Abstract
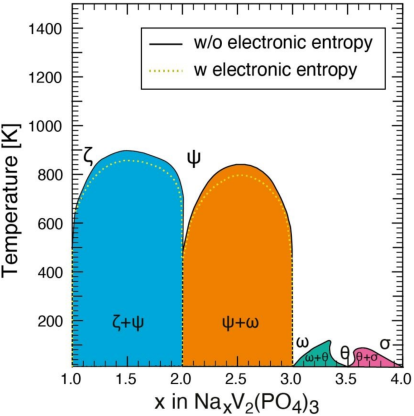
We elucidate the thermodynamics of sodium (Na) intercalation into the sodium super-ionic conductor (NaSICON)-type electrode, NaxV2(PO4)3, for promising Na-ion batteries with high-power density. This is the first report of a computational temperature-composition phase diagram of the NaSICON-type electrode NaxV2(PO4)3. Based on our computational data, we identify a thermodynamically stable phase with a composition of Na2V2(PO4)3 and describe its structural features. We also identify another metastable configuration that can occur at room temperature, namely Na3.5V2(PO4)3. We unveil the crystal-structure and the electronic-structure origins of the ground-state compositions associated with specific Na/vacancy arrangements, which are driven by charge orderings on the vanadium sites. These results are significant for the optimization of high-energy and power density electrodes for sustainable Na-ion batteries.