Abstract
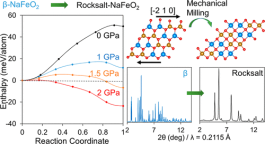
We report the synthesis of cation-disordered rocksalt NaMO2 (M = 3d transition metal), i.e., NaFeO2−δ (δ ∼ 0.18), for the first time via a martensitic-like phase transformation from β-NaFeO2. This disordered rocksalt NaFeO2−δ does not exhibit long-range cation ordering, as evidenced by synchrotron X-ray diffraction, neutron diffraction, and transmission electron microscopy analyses. Pair distribution function analysis revealed that although the cation sublattice mostly preserved the rocksalt symmetry, O atoms were significantly displaced to accommodate the different Na+–O, Fe2+–O, and Fe3+–O bond lengths (∼2.4, 2.1, and 2 Å, respectively). In addition, we confirmed the presence of O vacancies and partial Fe reduction from 3+ to 2+ in NaFeO2−δ based on 57Fe Mössbauer and X-ray absorption spectroscopy analyses. Controlled experiments revealed that three factors are essential for the successful synthesis of the rocksalt structure: (1) an appropriate precursor structure, (2) the application of a large stress by high-energy milling, and (3) the creation of O vacancies via carbon reduction. Density functional theory calculations demonstrated that these factors substantially affect the energetics of the minimum-energy reaction path of the phase transformation, making the highly metastable disordered rocksalt phase accessible. Our work suggests the effectiveness of mechanical milling to access metastable phases that are structurally related to the precursors via a shearing transformation. Utilizing this mechanism, we were able to predict and synthesize another cation-disordered rocksalt structure, NaMnO2, and explain the syntheses of several other related materials.