Abstract
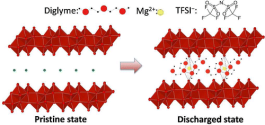
Functional multivalent intercalation cathodes represent one of the largest hurdles in the development of Mg batteries. While there are many reports of Mg cathodes, many times the evidence of intercalation chemistry is only circumstantial. In this work, direct evidence of Mg intercalation into a bilayer structure of V2O5·nH2O xerogel is confirmed, and the nature of the Mg intercalated species is reported. The interlayer spacing of V2O5·nH2O contracts upon Mg intercalation and expands for Mg deintercalation due to the strong electrostatic interaction between the divalent cation and the cathode. A combination of NMR, pair distribution function (PDF) analysis, and X-ray absorption near edge spectroscopy (XANES) confirmed reversible Mg insertion into the V2O5·nH2O material, and structural evolution of Mg intercalation leads to the formation of multiple new phases. Structures of V2O5·nH2O with Mg intercalation were further supported by the first principle simulations. A solvent cointercalated Mg in V2O5·nH2O is observed for the first time, and the 25Mg magic angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy was used to elucidate the structure obtained upon electrochemical cycling. Specifically, existence of a well-defined Mg–O environment is revealed for the Mg intercalated structures. Information reported here reveals the fundamental Mg ion intercalation mechanism in a bilayer structure of V2O5·nH2O material and provides insightful design metrics for future Mg cathodes.