Abstract
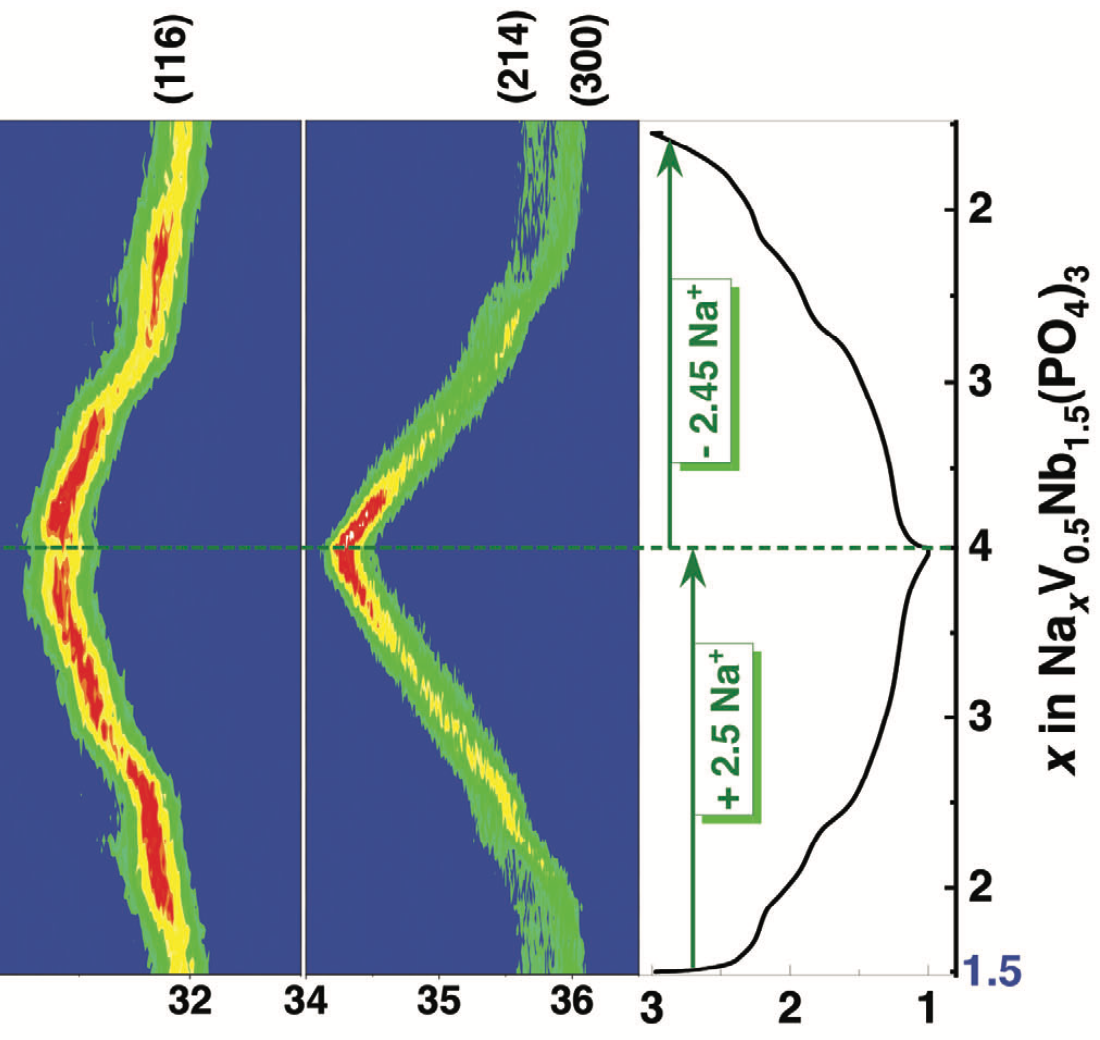
Multi-electron NAtrium SuperIonic CONductor (NASICON)-Nb2(PO4)3 (N0NbP) is an attractive Na-ion battery anode, owing to its low intercalation voltage (1.4 V vs Na+/Na0) and high capacity (≈150 mAh g−1). However, it suffers from poor capacity retention due to structural degradation. To overcome this issue, extra Na+ ions are introduced at the Na(1) sites, via V3+ substitution, which can act as stabilizing agents to hold lantern units together during cycling, producing NASICON-Na1.5V0.5Nb1.5(PO4)3 (N1.5VNbP). The N1.5VNbP anode exhibits reversible capacities of ≈140 mAh g−1 at 1.4 V versus Na+/Na0 through Nb5+/Nb4+/Nb3+ and V3+/V2+ redox activities. The extra Na+ ions in the framework forms a complete solid-solution during Na (de)intercalation and enhances sodium diffusivity, in agreement with first-principles calculations. Further, N1.5VNbP demonstrates extraordinary cycling (89% capacity retention at 5C after 500 cycles) and rate performances (105 mAh g−1 at 5C). Upon pairing the N1.5VNbP anode with the NASICON-Na3V2(PO4)3 cathode, the full Na-ion cell delivers a remarkable energy density of 98 Wh kg−1 (based on the mass of anode and cathode) and retains 80% of its capacity at 5C rate over 1000 cycles. The study opens new possibilities for enhancing the electrochemical performance of NASICON anodes via chemical and structural modulations.