Abstract
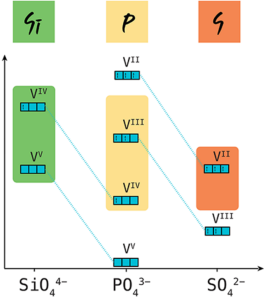
Polyanion-based electrode materials are important for rechargeable sodium(Na)-ion batteries owing to their structural stability and their high redox voltage. The redox voltages and gravimetric capacities of polyanion electrodes can be tuned by the choice of transition metal and/or polyanion groups. In this work we explore the effect of changing polyanion groups on the redox voltages and the gravimetric capacities of polyanion electrodes. Using first-principles calculations, we examine the influence of polyanionic substitutions on the stability and electrochemical behavior of the Na3V2(PO4)3 electrode material, which adopts the prototype structure of the natrium superionic conductor (NaSICON). Starting from the Na3V2(PO4)3 structure, we explore the partial or total substitution of PO43– groups by SiO44– or SO42– moieties, unveiling the uncharted multicomponent Na–V–P–(Si/S)–O phase diagram. We show that small amounts of SiO44– can activate the VV/IV redox couple, which is not experimentally accessible in the PO43– NaSICON analogue, thereby increasing the average Na intercalation voltage. In the case of SO42– substitution, we observe an increase in voltage of each V redox couple (i.e., VIII/II, VIV/III, and VV/IV) but at the expense of the maximum amount of Na+ intercalated. We show that exploiting the varying inductive effects of different polyanion groups can be effective for tuning the energy density of NaSICON electrode materials.