Abstract
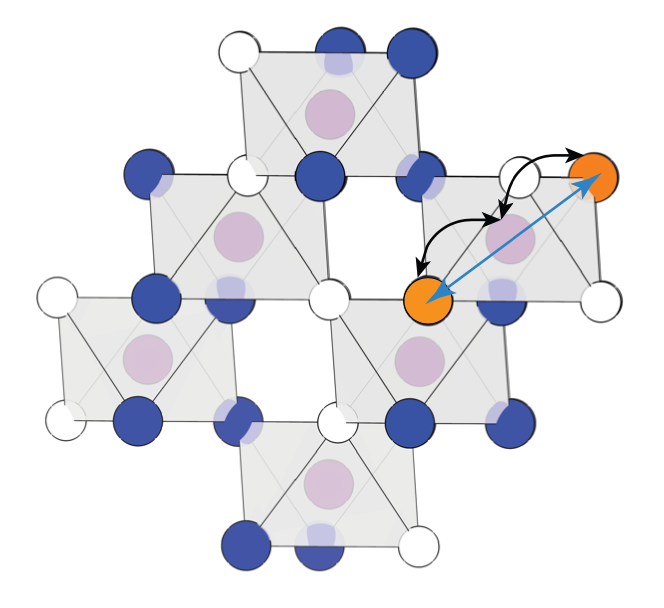
The development of high-performance sodium (Na) ion batteries requires improved electrode materials. The energy and power densities of Na superionic conductor (NaSICON) electrode materials are promising for large-scale energy storage applications. However, several practical issues limit the full utilization of the theoretical energy densities of the NaSICON electrodes. A pressing challenge lies in the limited sodium extraction in low Na content NaSICONs, e.g., Na1VIVVIV(PO4)3 ↔ VVVIV(PO4)3 + e– + Na+. Therefore, it is important to quantify the Na-ion mobility in a broad range of NaSICON electrodes. Using a kinetic Monte Carlo approach bearing the accuracy of first-principles calculations, we elucidate the variability of Na-ion transport vs Na content in three important NaSICON electrode materials, NaxTi2(PO4)3, NaxV2(PO4)3, and NaxCr2(PO4)3. We show Na+ transport in NaSICON electrode materials is almost entirely determined by the local electrostatic and chemical environment set by the transition metals and the polyanionic scaffold. The competition with the ordering-disordering phenomena of Na vacancies also plays a role in influencing Na transport. We identify the Na content providing the highest room-temperature diffusivities in these electrodes, i.e., Na2.7Ti2(PO4)3, Na2.9V2(PO4)3, and Na2.6Cr2(PO4)3. We link the variations in the Na+ kinetic properties by analyzing the competition of ligand field stabilization transition metal ions and their ionic radii. We interpret the limited Na extraction at x = 1 observed experimentally by gaining insights into the local Na vacancy interplay. Targeted chemical substitutions of transition metals disrupting local charge arrangements will be critical to reducing the occurrence of strong Na+-vacancy orderings at low Na concentrations, thus expanding the accessible capacities of these electrode materials.