Abstract
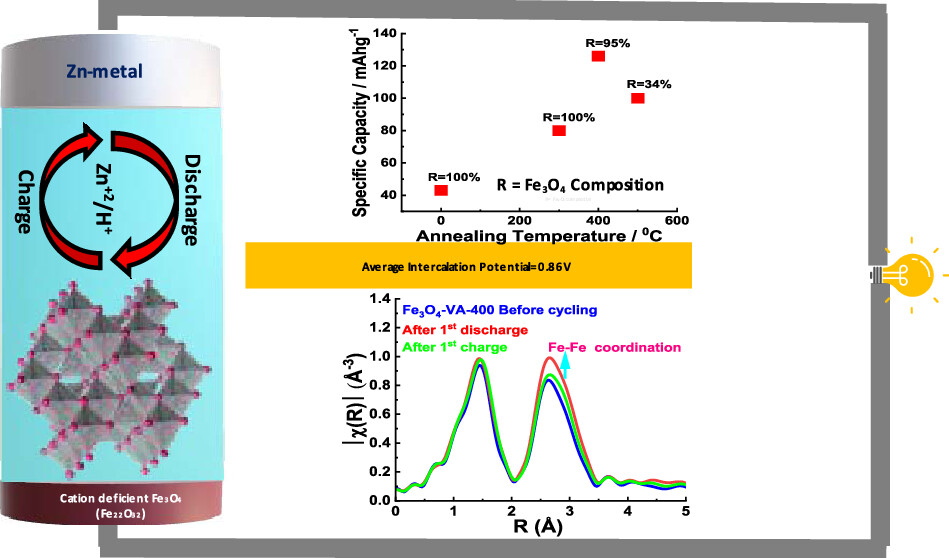
Zinc-ion aqueous battery (ZIAB) is a promising low-cost alternative, especially for large-scale energy storage applications, e.g., electric grid. The major challenge in ZIABs is the lack of suitable cathode materials, which can intercalate Zn2+ ions reversibly over long periods of time. Apart from the availability of suitable cathodes, gaps persist in our fundamental understanding of the mechanisms involved in energy storage in ZIABs. Herein, magnetite (Fe3O4) is explored as an alternative cathode for ZIAB. The magnetite cathode is separated from the Zn foil anode by a 1 M aqueous ZnSO4 electrolyte. Annealing of Fe3O4 at different temperatures (under moderate vacuum) led to the coexistence of mixed Fe3O4 and Fe2O3 phases as well as changes in the concentration of cation vacancy. Due to the optimal concentration of both cationic vacancies in Fe3O4 and the presence of the Fe2O3 phase, the Fe3O4 sample annealed at 400 °C exhibited the best electrochemical performance, with the highest initial discharge capacity of ∼212 mAh g–1. Detailed experimental and theoretical investigations reveal that the high capacity in the initial cycle, in addition to Zn ions, results from the coinsertion of H+ into the cation-deficient Fe3O4. Additionally, both experiment and theoretical calculations show the formation of Zn4SO4(OH)6.4H2O as a byproduct on the cathode surface, which is a direct consequence of H+ coinsertion into the cation-deficient Fe3O4.