Abstract
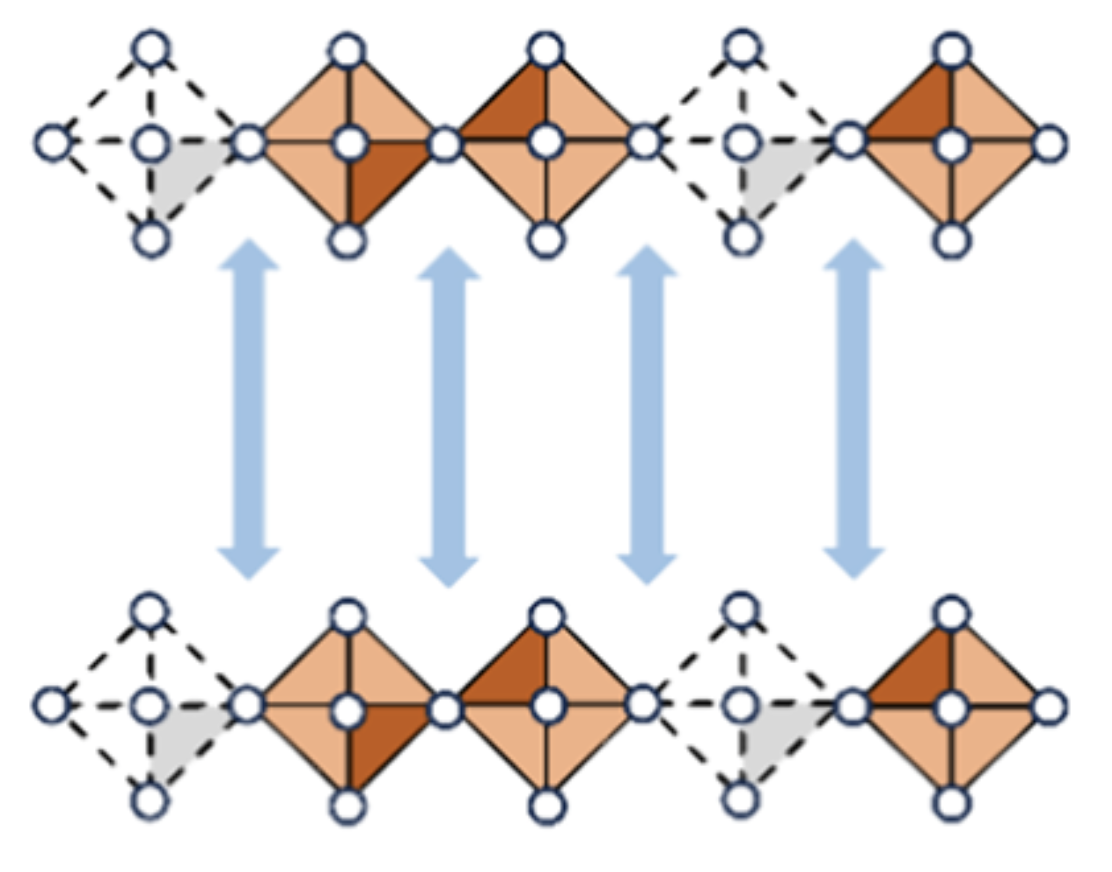
Two-dimensional (2D) hybrid iodide perovskites, (R-NH3)2MI4 and (H3N–R–NH3)MI4 (R = alkyl group; M = divalent metal ion), are promising materials for optoelectronics. Traditionally, these compounds contain Pb2+ and Sn2+ ions in the M-site; however, concerns over the toxicity of Pb2+ and the instability of Sn2+ ions have driven interest in Bi3+ halide-based alternatives. This study reports two Dion-Jacobson type, vacancy-ordered 2D Bi–I perovskites: (H2DAC)Bi2/3□1/3I4, with vacancy in every third metal site and (H2DAP)BiBi1/2□1/2I3·(I3)1/2, with vacancy in every second metal site (H2DAC = trans-1,4-diammoniumcyclohexane, H2DAP = 1,5-diammoniumpentane, and □ = vacancy). The band gaps of (H2DAC)Bi2/3□1/3I4 and (H2DAP)Bi1/2□1/2I3·(I3)1/2 are 2.11 and 1.97 eV, respectively─both narrower than that of Pb2+-based analogue (H2DAC)PbI4 (2.36 eV). These compounds show a positive photoresponse under light exposure, with the highest response observed in the case of (H2DAP)Bi1/2□1/2I3·(I3)1/2. This enhancement is attributed to the presence of I3– ions, which not only cross-link the perovskite layers and stabilize the H2DAP cation in its zigzag conformation but also contribute to the frontier orbitals. DFT calculations corroborate these experimental results. Overall, this study introduces an approach for synthesizing hybrid Bi(III)-I perovskites, which may be further investigated as lead-free optoelectronic materials, including in perovskite photovoltaics.