Abstract
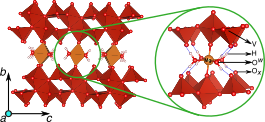
Cointercalation is a potential approach to influence the voltage and mobility with which cations insert in electrodes for energy storage devices. Combining a robust thermodynamic model with first-principles calculations, we present a detailed investigation revealing the important role of H2O during ion intercalation in nanomaterials. We examine the scenario of Mg2+ and H2O cointercalation in nanocrystalline Xerogel-V2O5, a potential cathode material to achieve energy density greater than Li-ion batteries. Water cointercalation in cathode materials could broadly impact an electrochemical system by influencing its voltages or causing passivation at the anode. The analysis of the stable phases of Mg-Xerogel V2O5 and voltages at different electrolytic conditions reveals a range of concentrations for Mg in the Xerogel and H2O in the electrolyte where there is no thermodynamic driving force for H2O to shuttle with Mg during electrochemical cycling. Also, we demonstrate that H2O shuttling with the Mg2+ ions in wet electrolytes yields higher voltages than in dry electrolytes. The thermodynamic framework used to study water and Mg2+ cointercalation in this work opens the door for studying the general phenomenon of solvent cointercalation observed in other complex solvent–electrode pairs used in the Li- and Na-ion chemical spaces.