Abstract
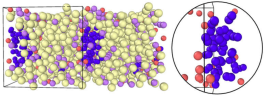
Liquid metals are being explored actively as candidates for plasma-facing components (PFCs) in fusion reactors. Recently, Li–Sn alloys have appeared as promising alternatives that could overcome some of the challenges faced by the well-studied liquid Li system, namely, a vapor pressure that limits the operating temperature and a high hydrogen isotope retention. However, only scarce data (experimental or theoretical) are available concerning the performance of Li–Sn alloys, specifically only for the compositions of Li30Sn70 and Li20Sn80, related to their bonding and retention of deuterium (D). Here, we present a comprehensive, first-principles molecular-dynamics study of static and dynamic properties of liquid Li30Sn70 at various D concentrations. We observe the formation of D2 gas bubbles for β in Li30Sn70D β greater than 22.5 along with Li segregation towards D2 bubbles. To understand the effect of Sn addition on D retention in Li–Sn alloys, we perform a thermodynamic evaluation of maximum D retention in Li-rich Li–Sn alloys. Overall, this work will provide useful data and guidance in the development of Li–Sn PFCs in fusion reactors.