Abstract
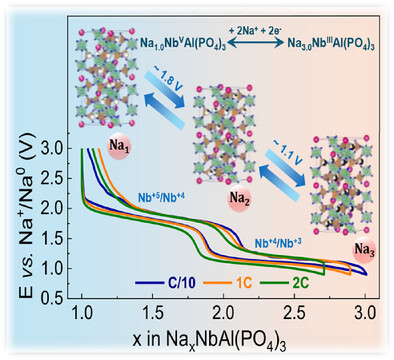
Natrium SuperIonic CONductor (NASICON)-Nb2(PO4)3 is regarded as a potential anode for sodium-ion batteries due to its higher storage capacity (~150 mAh g-1) stemming from two-electron Nb5+/Nb4+/Nb3+ redox. However, the reversibility of Nb5+/Nb4+/Nb3+ redox is limited by its structural degradation. Herein, we unveil highly reversible Nb5+/Nb4+/Nb3+ redox reactions in bulk NASICON-NaNbAl(PO4)3 (NaNbAl) anode. The introduction of Na-ions via Al3+ substitution stabilizes the NASICON framework and activates distinct Nb redox couples with Na (de)intercalation, as demonstrated via our density functional theory-based calculations. This bulk anode delivers capacities up to 90 mAh g-1 at 5C with 86% retention after 1000 cycles. More significantly, we capture rapid sodium ion (de)intercalation in NaNbAl at high current rates using operando synchrotron X-ray diffraction which is in agreement with the calculated low migration barriers associated with Na motion within the structure. A full Na-ion cell (Na4V2(PO4)3||NaNbAl) achieves an energy density of 201 Wh kg-1 (based on cathode mass) and retains 84% capacity over 200 cycles at 1C. This study opens new avenues for realizing reversible Nb5+/Nb4+/Nb3+ redox in bulk NASICON anodes with suitable chemical substitutions.