Abstract
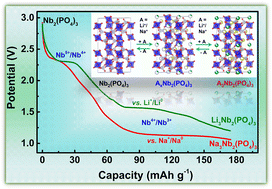
Sodium superionic conductor (NASICON)-type materials are widely explored as Li- and Na-ion cathodes and solid-state electrolytes but are largely ignored as anodes due to their lower capacities and higher intercalation voltages, which reduce the overall energy densities of Li- and Na-ion batteries (LIBs and SIBs). Herein, we unveil high capacity multi-redox empty NASICON-Nb2(PO4)3 as a potential anode material for LIBs and SIBs, which reversibly delivers 167 and 150 mA h g−1 at the average voltages of 1.86 V vs. Li+/Li0 and 1.46 V vs. Na+/Na0, respectively. The Li and Na intercalation reactions proceed via multiple phase transitions, leading to short-range ordered Li3Nb2(PO4)3 and triclinic (P1) Na3Nb2(PO4)3, as revealed by in situ X-ray diffraction studies. Our density functional theory calculations are also in agreement with the in situ measurements in predicting a stable Na3Nb2(PO4)3 composition in the Na–Nb2(PO4)3 pseudo-binary system. X-ray absorption spectroscopy confirms the participation of multi-redox Nb5+/Nb4+/Nb3+ couples. The Nb2(PO4)3 anode delivers capacities greater than 124 and 106 mA h g−1 at 1C rate in Li and Na cells, respectively. Pairing Nb2(PO4)3 with suitable cathodes and electrolytes can lead to high energy density batteries.