Abstract
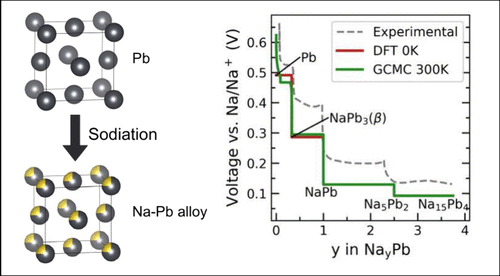
Metals, such as tin, antimony, and lead (Pb) have garnered renewed attention for their potential use as alloyant-negative electrode materials in sodium (Na)-ion batteries (NIBs). Despite Pb’s toxicity and its high molecular weight, lead is one of the most commonly recycled metals, positioning Pb as a promising candidate for a cost-effective, high-capacity anode material. Understanding the miscibility of Na into Pb is crucial for the development of high-energy density negative electrode materials for NIBs. Using a first-principles multiscale approach, we analyze the thermodynamic properties and estimate the Na-alloying voltage of the Na−Pb system by constructing the compositional phase diagram. In the Na−Pb system, we elucidate the phase boundaries of important phases, such as Pb-rich face-centered cubic and β-NaPb3, thereby improving our understanding of the phase diagram of the Na−Pb alloy. Due to the strong ordering tendencies of the Na−Pb intermetallics (such as NaPb, Na5Pb2, and Na15Pb4), we do not observe any solid-solution behavior at intermediate and high Na concentrations.