Abstract
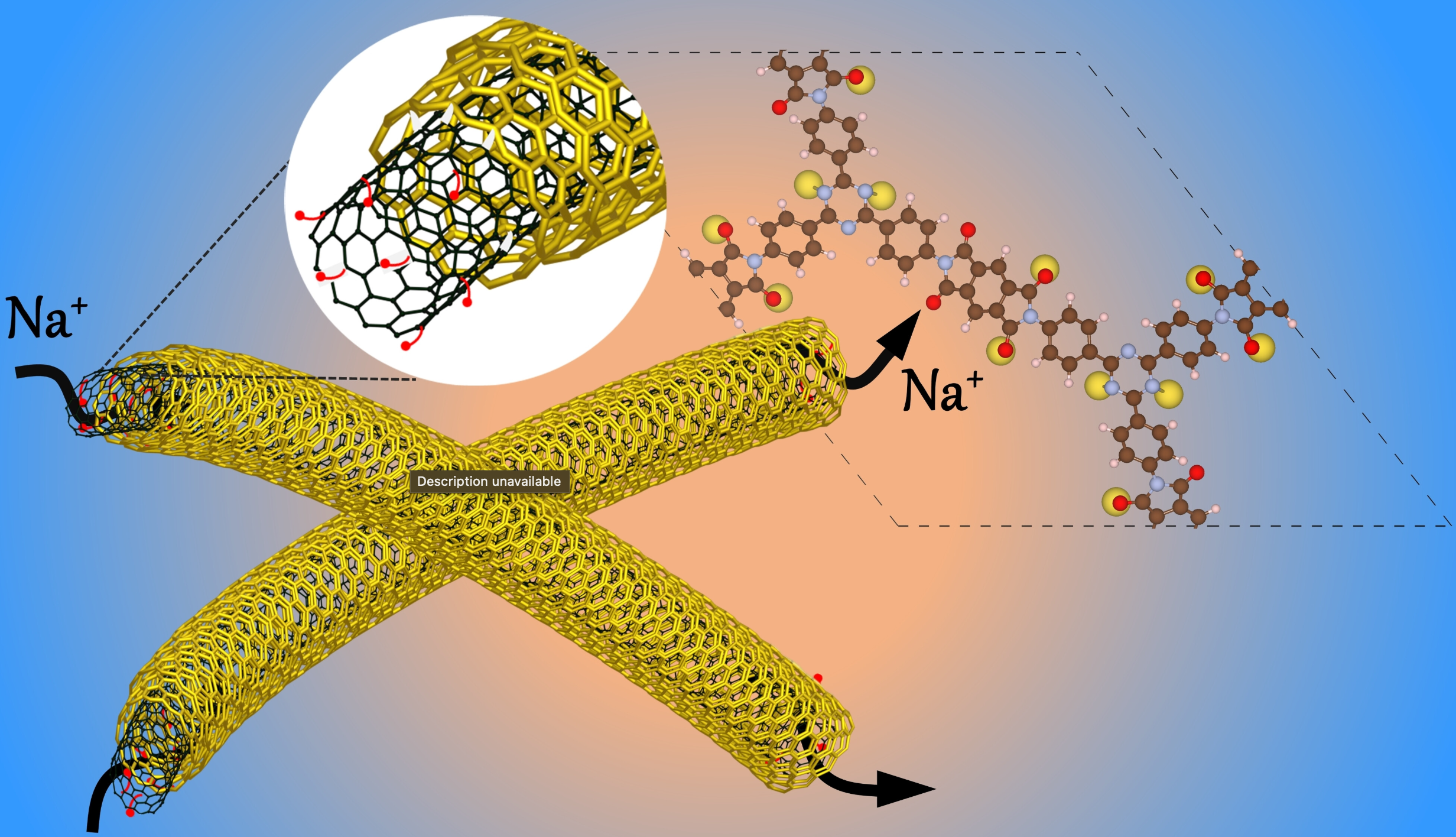
Redox-active covalent organic frameworks (COFs) with metal binding sites are increasingly recognized for developing cost-effective, eco-friendly organic electrodes in rechargeable energy storage devices. Here, we report a microwave-assisted synthesis and characterization of a triazine-based polyimide COF that features dual redox-active sites (−C=O from pyromellitic and −C=N− from triazine) and COF@CNT nanocomposites (COF@CNT-X, where X=10, 30, and 50 wt % of NH2-MWCNT) formed through covalent linking with amino-functionalized multiwalled carbon nanotubes. These composites are evaluated as cathode materials for the sodium-ion batteries (SIBs). The amine functionalization renders the covalent bond between COF and CNT, improving electronic conductivity, structural rigidity, and long-term stability. The interfacial growth of COF layers on CNTs increases accessible redox-active sites, enhancing sodium diffusion kinetics during sodiation/desodiation. The COF@CNT-50 composite exhibits outstanding Na+ ion storage performance (reversible capacity of 164.3 mAh g−1 at 25 mA g−1) and excellent stability over 1000 cycles at ambient temperature. At elevated temperature (65 °C), it also maintains good capacity and cycle stability. Ex situ XPS analysis confirms the importance of dual active sites in the Na+ diffusion mechanism. Density functional theory (DFT) calculations reveal insights into Na+ binding sites and corresponding binding energies into COF structure, elucidating the experimental storage capacity and voltage profile.