Abstract
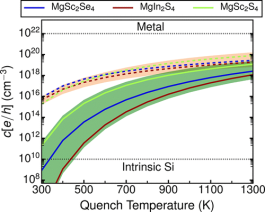
Close-packed chalcogenide spinels, such as MgSc2Se4, MgIn2S4, and MgSc2S4, show potential as solid electrolytes in Mg batteries, but are affected by non-negligible electronic conductivity, which contributes to self-discharge when used in an electrochemical storage device. Using first-principles calculations, we evaluate the energy of point defects as a function of synthesis conditions and Fermi level to identify the origins of the undesired electronic conductivity. Our results suggest that Mg-vacancies and Mg-metal antisites (where Mg is exchanged with Sc or In) are the dominant point defects that can occur in the systems under consideration. While we find anion-excess conditions and slow cooling to likely create conditions for low electronic conductivity, the spinels are likely to exhibit significant n-type conductivity under anion-poor environments, which are often present during high-temperature synthesis. Finally, we explore extrinsic aliovalent doping to potentially mitigate the electronic conductivity in these chalcogenide spinels. The computational strategy is general and can be easily extended to other solid electrolytes (and electrodes) to aid the optimization of the electronic properties of the corresponding frameworks.