Abstract
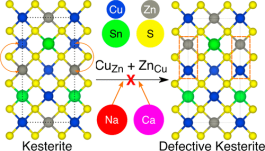
A pathway to improve the efficiency of Cu2ZnSnS4 (CZTS)-based solar cells, which form an important class of beyond-Si, thin-film photovoltaic technology, is the employment of sustainable isovalent dopants (substituting for Cu and Zn) to suppress formation of disorder-inducing, performance-limiting antisite defects. Using calculations based on density functional theory, we examine the influence of Na and Ca as isovalent dopants for Cu and Zn, respectively, on defect formation, thermodynamic stability, and electronic properties of CZTS. On the basis of defect formation energies, we find that Na-doping should be feasible within CZTS while the incorporation of Ca will be difficult. Importantly, both Na and Ca effectively mitigate formation of antisites that cause disorder, if incorporated within the CZTS structure, across doping and Cu-chemical-potential conditions. Thermodynamically, doping high concentrations of Na into CZTS will result in phase separation between CZTS and Na2ZnSnS4 domains, whereas large additions of Ca will lead to formation of other secondary phases, such as Cu2SnS3 and CaS. With respect to electronic properties, Na (Ca) doping should cause a significant (marginal) increase in the band gap of kesterite CZTS. Overall, we suggest low Na-doping in CZTS as a promising pathway to improve performance of CZTS-based solar cells.