Abstract
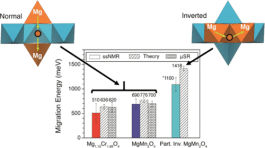
Mg batteries utilizing oxide cathodes can theoretically surpass the energy density of current Li-ion technologies. The absence of functional devices so far has been ascribed to impeded Mg2+ migration within oxides, which severely handicaps intercalation reactions at the cathode. Broadly, knowledge of divalent cation migration in solid frameworks is surprisingly deficient. Here, we present a combined experimental and theoretical study of Mg migration within three spinel oxides, which reveal critical features that influence it. Experimental activation energies for a Mg2+ hop to an adjacent vacancy, as low as ∼0.6 eV, are reported. These barriers are low enough to support functional electrodes based on the intercalation of Mg2+. Subsequent electrochemical experiments demonstrate that significant demagnesiation is indeed possible, but the challenges instead lie with the chemical stability of the oxidized states. Our findings enhance the understanding of cation transport in solid structures and renew the prospects of finding materials capable of high density of energy storage.